With vaccination campaigns taking place worldwide to end the COVID-19 pandemic, everyone has different questions about various COVID-19 vaccines. Here are answers to some of the most common questions.
1. What COVID-19 vaccines are available?
Seven COVID-19 vaccines are available to the public in more than 75 countries through various forms of approval and emergency authorization. The approval process differs around the world, so availability of vaccines will depend on where you reside.
The first vaccine authorizations occurred in Russia with the Sputnik V vaccine from the Gamaleya Research Institute and in China with vaccines from CanSinoBIO, Sinovac, and Sinopharm. These authorizations occurred before large, phase 3 clinical trials were completed. Currently in the United States, two vaccines are authorized for use from Pfizer/BioNTech and Moderna and both are authorized in various other countries worldwide. AstraZeneca’s vaccine was first approved in the United Kingdom and has since gained authorization across Europe and in several other countries. Johnson & Johnson and Novavax COVID-19 vaccines appear next in line for authorization.
Expeditious worldwide vaccine coverage with multiple COVID-19 vaccine options is an important public health goal. While global vaccine supplies remain limited, however, choice of vaccine cannot be guaranteed even if residing in a country where several vaccines have received authorization.
2. What are the differences and similarities between the various COVID-19 vaccines?
The primary difference between vaccines is whether they are made from a whole virus (either SARS-CoV-2 or a viral vector), the genetic material from the virus (DNA or mRNA), or parts of the virus (protein subunit). The Sinovac and Sinopharm vaccines are whole virus vaccines made of inactivated SARS-CoV-2 virus. The AstraZeneca, Sputnik V, CanSinoBIO, and Johnson & Johnson vaccines are viral-vectored vaccines using different strains of adenovirus encoding the SARS-CoV-2 spike gene. The Pfizer/BioNTech and Moderna vaccines are mRNA vaccines that carry mRNA instructions to make the SARS-CoV-2 spike protein surrounded in a lipid nanoparticle. Finally, Novavax will likely be the first protein subunit vaccine authorized for use.
Other differences include dosing schedules and storage requirements. For example, the CanSinoBIO and Johnson & Johnson vaccines are authorized for single dose administration, while the others are authorized as a two-dose vaccine series. For vaccines requiring two doses, the recommended interval between doses varies from 14 to 56 days. Storage temperature requirements also differ, with the Pfizer/BioNTech vaccine requiring ultra-cold freezer temperature (minus 70 degrees Celsius), making distribution more difficult.
There are also similarities between the vaccines currently authorized for use. They are all administered via intramuscular injection into the deltoid muscle of the arm. They all elicit antibody and T cell responses, which may cause pain and/or swelling at the injection site and sometimes systemic symptoms such as fever, swollen glands, myalgia, and headache. Systemic symptoms appear more common after the second vaccine dose.
3. Is one vaccine better than another?
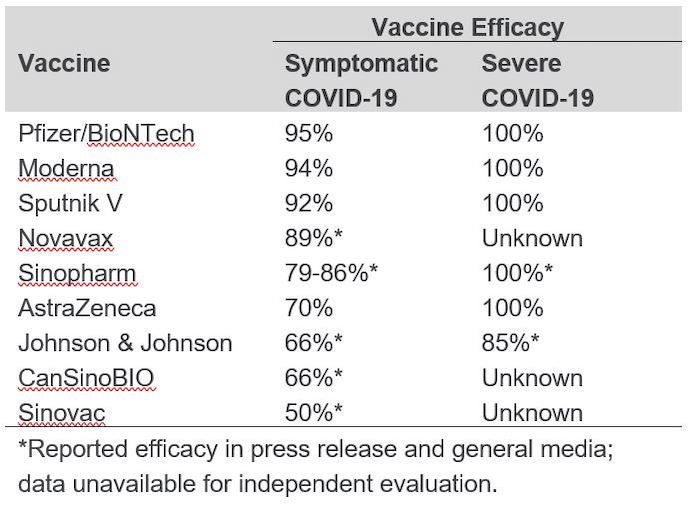
To date, there have been no head-to-head comparisons of the vaccines in clinical trials, and it is not fair to make a comparison of vaccine efficacy based on individual placebo-controlled trials. Differences in study populations, circulating variant strains at study sites, outcomes measured, and timing of evaluation could all contribute to varying degrees of vaccine efficacy — even among different trials of the same vaccine. The overall vaccine efficacy reported in phase 3 trials published in peer reviewed journals varies from 70 to 95 percent, and each vaccine evaluated was found to prevent severe disease and COVID-19-related death. (See table below for reported efficacy of each.)
4. Are the vaccines effective against SARS-CoV-2 variants?
Whether the currently authorized vaccines are effective against variants of the SARS-CoV-2 virus is an area of active investigation, and we continue to learn more each day. Early studies suggest that the Pfizer/BioNTech and Moderna mRNA vaccines elicit antibodies capable of neutralizing the notable circulating variants (B.1.1.7, B.1.351, and P.1), though antibody titers against B.1.351 may be lower. However, clinical trials based in South Africa, where the B.1.351 variant is widespread, appear to indicate that some vaccines may be less effective against this variant. A trial of the Johnson & Johnson vaccine showed 57 percent efficacy in South Africa compared to 72 percent efficacy in the United States. And a small trial of the AstraZeneca vaccine failed to demonstrate protection, prompting South Africa to halt the planned rollout of this vaccine.
Continued surveillance for SARS-CoV-2 variants and clinical trials in different sites throughout the world will help to identify which vaccines are more (or less) effective against SARS-CoV-2 variants and in what geographic locations. If a variant strain emerges that is able to evade immunity, it may be necessary to receive a booster aimed at the variant. Toward that end, Moderna has already committed to testing second generation vaccines using SARS-CoV-2 spike mRNA with variant strain mutations.
5. Who should not be vaccinated?
Presently, children under the age of 16 should not be vaccinated. The only COVID-19 vaccine currently available to adolescents is the Pfizer/BioNTech vaccine, which is authorized for people at least 16 years of age. All other vaccines are authorized for adults 18 years or older. Clinical trials that include children have been initiated, but for now children under 16 years of age should not be vaccinated with any COVID-19 vaccine.
The only medical contraindication to COVID-19 vaccination is a severe allergic reaction to a previous vaccine dose or any vaccine component (ingredients in mRNA vaccines can be found from CDC). For the mRNA vaccines, history of allergy to polysorbate is also considered a contraindication because of a cross-reactive immune response to polyethylene glycol in the lipid nanoparticle. Any concern about allergy to vaccination should prompt discussion with a healthcare provider.
Precautions to vaccination may vary regionally and differ for each vaccine. The World Health Organization suggests delayed vaccination in people who are pregnant or breastfeeding, though other agencies including the American College of Obstetricians and Gynecologists (ACOG) state that vaccination should not be withheld from pregnant people. Considerations may include risk of exposure, level of COVID-19 in the community, risk and severity of maternal COVID-19 including risks to the fetus, and safety and efficacy of the vaccine. Medical conditions that may warrant precaution include persons with immunocompromise, autoimmune disorders, or history of Guillain-Barre syndrome or Bell’s palsy. Vaccine precautions are not contraindications; however, they should prompt discussion with a healthcare provider.
Vaccination should also be offered to persons with a history of confirmed COVID-19, though this guidance may also differ regionally and depend on vaccine supply. Prior to vaccination, people with a recent diagnosis of COVID-19 should be recovered from illness and meet all criteria for discontinuing isolation. A 90-day deferral of vaccination is suggested for patients with COVID-19 who received either monoclonal antibody or convalescent plasma therapy as a precautionary measure to avoid possible treatment-related interference with the vaccine-induced immune response.
Vaccination is not recommended as post-exposure prophylaxis for people with a known exposure as it is unlikely to provide protection. People with a known exposure to someone with COVID-19 should delay vaccination until their quarantine period is over to avoid potential transmission.
The general guidance is to get whichever COVID-19 vaccine is available whenever it is offered, while continuing mask wearing, physical distancing, and frequent handwashing post vaccination. At present, no specific vaccine is recommended over another.